| 215-792-6411 | |
| 848-459-6295 |
Listen
Communicate
Deliver
Our Services
The founders of NiRACLE believe Process Analytical Technology (PAT) and Quality by Design (QbD) represent the future of the pharmaceutical industry. As a knowledge-based company, NiRACLE will serve clients in these areas. As implied by its name and logo, NiRACLE is specialized in Near Infrared (NIR) technologies including the conventional NIR and NIR chemical imaging. NiRACLE is also specialized in multivariate data analysis and modeling. The combination of both forms a powerful tool that is badly needed in pharmaceutical research and development.
Although NIR and multivariate data analysis are becoming popular in the pharmaceutical industry, along with PAT and QbD, the sciences behind them are not well publicized and understood. This is due to them not having been part of the core curriculum of chemistry professionals for a long time.
| METHOD DEVELOPMENT |
 NiRACLE assists clients in developing NIR-based methods for both GMP and non-GMP applications. Non-GMP NIR methods take advantage of NiRACLE’s proprietary modeling method and are intended for supporting early stage formulation development and late stage commercial production scale up. GMP NIR methods are for tablet content uniformity testing and can be used for supporting NDA/ANDA filings. NiRACLE also assists clients in developing real-time process monitoring methods for various unit operations including blending, film coating and granulation. The services also include experimental design, method validation and method transfer. NiRACLE assists clients in developing NIR-based methods for both GMP and non-GMP applications. Non-GMP NIR methods take advantage of NiRACLE’s proprietary modeling method and are intended for supporting early stage formulation development and late stage commercial production scale up. GMP NIR methods are for tablet content uniformity testing and can be used for supporting NDA/ANDA filings. NiRACLE also assists clients in developing real-time process monitoring methods for various unit operations including blending, film coating and granulation. The services also include experimental design, method validation and method transfer. |
| PROCESS VALIDATION |
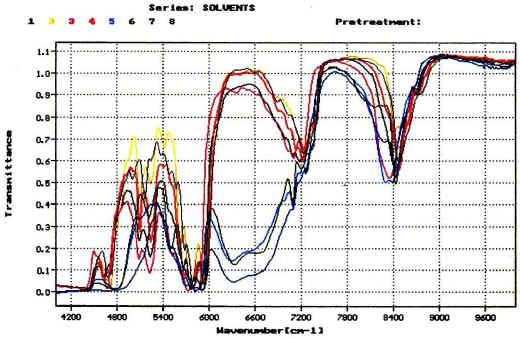
 NiRACLE is specialized in multivariate data analysis, which plays a critical role in today’s process validation. NiRACLE works with clients in selecting suitable process monitoring methods and designing a comprehensive sampling plan. NiRACLE’s technology allows for the fast analysis of thousands of process related samples for mapping the manufacturing processes. Our services are extremely cost-effective and the results will give clients a much better understanding of their manufacturing processes and products. NiRACLE is specialized in multivariate data analysis, which plays a critical role in today’s process validation. NiRACLE works with clients in selecting suitable process monitoring methods and designing a comprehensive sampling plan. NiRACLE’s technology allows for the fast analysis of thousands of process related samples for mapping the manufacturing processes. Our services are extremely cost-effective and the results will give clients a much better understanding of their manufacturing processes and products. |
| PHARMACEUTICAL PRODUCT/PROCESS TROUBLESHOOTING |
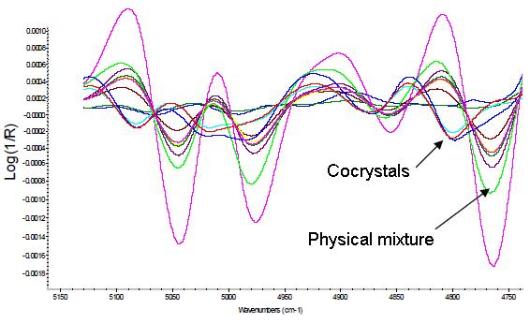
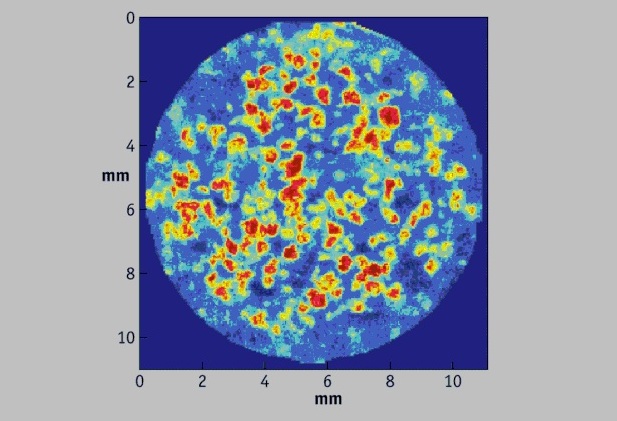 Using NIR chemical imaging and multivariate data analysis tools, NiRACLE can assist clients in troubleshooting product and process related problems. Examples include content uniformity related failures, dissolution problems, polymorph conversions during manufacturing and stability studies, drug release related issues for prolonged release formulations, and problems related to fluid-bed wet granulation processes. Using NIR chemical imaging and multivariate data analysis tools, NiRACLE can assist clients in troubleshooting product and process related problems. Examples include content uniformity related failures, dissolution problems, polymorph conversions during manufacturing and stability studies, drug release related issues for prolonged release formulations, and problems related to fluid-bed wet granulation processes. |
| RAW MATERIAL IDENTIFICATION BY NIR |
 NiRACLE can help clients in developing a NIR-based raw material identification system to replace the conventional wet chemistry methods. This approach is cost effective, fully GMP compliant, and accepted by the regulatory agencies. NiRACLE can help clients in developing a NIR-based raw material identification system to replace the conventional wet chemistry methods. This approach is cost effective, fully GMP compliant, and accepted by the regulatory agencies. |
| USER TRAINING |
 NiRACLE provides training in basic and advanced NIR/NIR chemical imaging related method development, which include proper use of instruments, experiment design and multivariate modeling. NiRACLE’s experts conduct the trainings through class-room presentations and real-life project related practices alike. NiRACLE provides training in basic and advanced NIR/NIR chemical imaging related method development, which include proper use of instruments, experiment design and multivariate modeling. NiRACLE’s experts conduct the trainings through class-room presentations and real-life project related practices alike. |
Case Studies
|
|
|
|||
|
|
||||
|
|
|
|||
|
|
||||
|
|
|
|||
|
|
||||
|
|
|
|||
|
|
||||
|
|
|
|||

| Home | Company | Services | Case Studies | Resources | Contact | News Releases
Copyright ©2019 Niracle, LLC . All rights reserved Privacy Statement | Terms and Conditiions |